Advocate Reaction: Thinking Out Loud
My favorite way to start a day is with a fresh coffee (iced of course) and a good article to read. I’m always excited when a new issue of The Advocate comes out from FARA – it’s a welcome change from the depressing, doomsday articles I always find in the newspapers. Per usual, the Summer 2015 issue did not disappoint, and I was rejuvenated reading Executive Director Jennifer Farmer’s article summarizing the research pipeline.
I didn’t realize FARA is funding 16 of 19 candidates on the list and advocating policy to advance research and drug development – that’s the definition of fundraising ROI! Jen’s article went on to summarize the active trials, with descriptions of what the study entails and links directly to the flyers. Did you know there are currently 3 active drug trials for FA and they each have a unique treatment approach? That’s a diversified portfolio! (OK, I promise I’m done with the finance references!)
When I looked at the new pipeline image, I got dizzy from all the colors and realized I might need a wider computer monitor… so many different scientific approaches… so many companies/groups involved… so much progress thus-far… AMAZING!!! Needless to say, I had a good day. Read Jen's article below.
![]()

The below article was originally published in the Summer 2015 edition of the FARA Advocate.
"Treatment Pipeline Progress and New Cliinical Trials"
By Jennifer Farmer, FARA Executive Director
As part of FARA’s website makeover we also had our pipeline image redesigned. You no longer need to tilt your head 90 degrees to read the bars! This new design allows us room for future growth of new mechanisms of action and lead candidates and makes it easier to track progress.
Down the left hand side you’ll find the categories of candidates based on mechanisms or approaches to treatment. Each candidate advances from the left to the right with various milestones identified across the top, from early research or discovery phase through clinical research phases to approved treatments.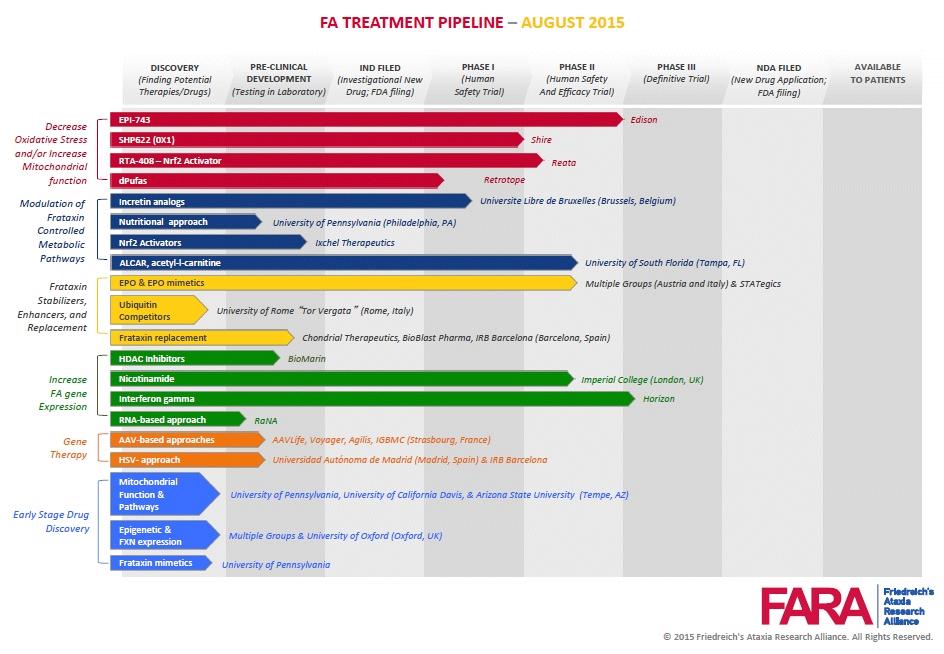
FARA is working with pharmaceutical and biotech companies and academic researchers who are advancing these lead candidates in a variety of ways.
• FARA facilitates introductions and develops collaborations between companies interested in working on FA and academic researchers and clinicians with expertise in the disease.
• FARA’s grant program has provided funding to 16 of the 19 candidates or drug screening programs listed.
• FARA’s grant program has funded research for new cell and animal models and other research tools that are used to test drugs in the pipeline.
• FARA provides funding and leadership for the Collaborative Clinical Research Network in FA which conducts research on the natural history, clinical measures, biomarkers, and quality of life, all of which are necessary to inform and expedite clinical trial design and execution.
• FARA’s Patient Registry is used to recruit individuals for clinical trials. When we are able to have individuals quickly identified for research studies we can shorten the time line of the study by months.
• FARA advocates for policy initiatives that provide greater awareness, financial resources or other support for research, drug development and regulatory flexibility and efficiencies for FA and other rare diseases.
As of July 1, 2015 we have three clinical trials and many clinical research studies actively recruiting and enrolling – read more below and click on the titles to link to the FARA website for more details, study site flyers and contact information.
• Horizon’s Actimmune Phase 3 Trial (STEADFAST) – The STEADFAST study opened enrollment in June 2015 at Children’s Hospital of Philadelphia and University of Iowa. This study is enrolling 90 individuals with Friedreich’s ataxia (FA) ages 10-25 years who are able to walk 25 feet with or without assistance. Additional study sites include University of Florida which opened enrollment on July 9th and University of California Los Angeles which is expected to open enrollment later in July. Also, in April, Horizon Pharma plc announced that they were granted Fast Track Designation from the FDA for ACTIMMUNE® (interferon gamma-1b) in the treatment of FA. This Fast Track Designation may be very helpful in shortening the timeline to approval of the drug for FA if the results of the clinical trial are favorable.
• Reata Phase 2 Trial for RTA 408 (MOXIe) – Enrolling individuals with FA ages 16-40 years who are able to perform 10-15 minutes of exercise on a recumbent exercise bike. We are pleased to announce that 16 patients have been enrolled to date in the MOXIe study. Reata is opening two additional eight patient cohorts to test additional doses between 20mg – 40mg of study drug. A third cohort of eight patients will be available at the following sites: University of Florida, The Ohio State University, University of South Florida, and Emory University. Once eight patients are enrolled in Cohort 3, an additional eight patients may be enrolled in Cohort 4 later this summer. Additional sites, which include University of California Los Angeles and Children’s Hospital of Philadelphia, will be available later this summer for Cohort 4.
• Cardiovascular Effects of Acetyl-L-Carnitine – Enrolling individuals with FA 18-80 years, at the University of South Florida, Tampa, FL
• University of Minnesota MRI Study – Enrolling individuals with FA ages 10 and over who have very early symptoms and/or are newly diagnosed or have had pre-symptomatic diagnosis at the University of Minnesota, Minneapolis, MN
• Weill Cornell Study of Cardiomyopathy –Enrolling individuals with FA 18-30 years at Weill Cornell, New York, NY
We are also happy to report that enrollment and the active study for the Phase I VP20629/SHP622 was completed at the end of June. No further subjects are needed. The sites and the company are now working to lock the database. Thank you to all who participated in this study.
As a community, by fundraising and participating in research, you are changing the profile of the treatment pipeline to add more bars and advance the existing ones. We are honored to steward the dollars you raise to fund good scientific research, and we are grateful for your time spent traveling to participate in clinical research. Thank you for your commitment on all fronts of FARA’s mission. Together we will treat and cure FA!
Click HERE to read all articles in the Summer 2015 edition of the Advocate. Past editions of the Advocate can be found HERE.
To receive future publications of the Advocate, please join FARA's mailing list.





