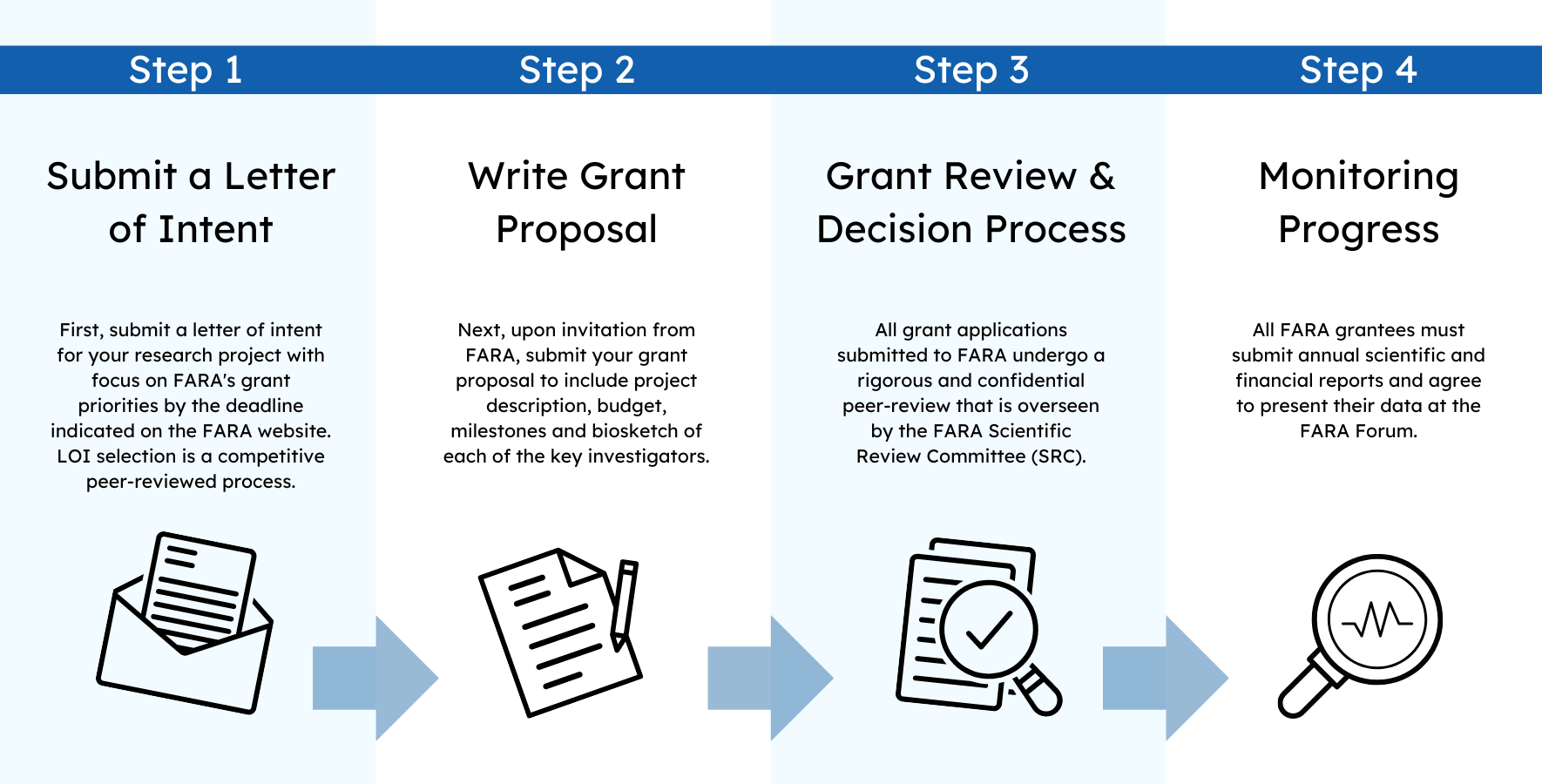
All applications must be submitted using FARA’s grant submission portal. This includes a lay summary of the project, budget and a timeline with detailed milestones. The project description, Data Management and Sharing Plan, curriculum vitae/biosketch of each of the key investigators, letters of support, and other relevant documents must be submitted as PDF files.
Click here for special application requirements for the Award for Innovative Mindset.
Project Description
The description of the project (excluding references to literature) should not exceed 10 pages, which includes the specific aims, background, preliminary data, and research plan, plus a brief statement on the suitability of the scientific environment and the availability of the necessary resources/equipment for the proposed research. This page limit excludes references and applies to grants less than $300,000. Larger grants do not have page number restrictions. All grant proposals must provide adequate detail for reviewer evaluation.
Data Management and Sharing Plan
FARA is committed to making the results and outputs of FARA-funded research available to researchers through effective and efficient data management and data sharing practices, and improving the reproducibility and reliability of research findings. Please read the FARA Notice for Data Management and Sharing. Under the DMS Notice, FARA requires researchers to prospectively plan for how scientific data will be preserved and shared through submission of a Data Management and Sharing Plan (DMS Plan). The DMS Plan will be reviewed as part of the FARA grant review process and upon FARA approval, FARA expects researchers and institutions to implement data management and sharing practices as described.
Budget
A detailed budget must be submitted with all proposals, including a justification of all requested expenses. Allowable expenses are: personnel costs/salary with fringe benefits, laboratory reagents and supplies, equipment, animal expenses, publication costs, investigator travel to meetings and conferences and to facilitate collaborations, patient expenses directly related to study and not reimbursable by third party insurers, and patient travel. Expenses that will not be awarded include: indirect costs/overhead, supplementing a postdoc fellowship/research award stipend with another FARA grant, memberships in scientific societies. Budget justification for investigator travel to meetings, conferences and to facilitate collaborations must include the destination, number of people traveling and dates or duration of stay for all anticipated travel, and clearly state how the travel is directly related to the proposed research. Budget limits for each grant type must be met. However, for General Research Grant applications, budgets exceeding 125,000/year will be considered if adequately justified, for example in the case of collaborations involving multiple PIs. The maximum direct salary that can be requested cannot exceed the NIH salary cap for 100% effort. The maximum allowable expense for travel cost is $3000/year. The max allowable expense for publication cost is $2000/year. The maximum allowable expense for data sharing and management cost is $3000/year.
For Postdoctoral Fellowships and Awards, the trainee is required to contribute at least 75% effort to the proposed work. The maximum direct salary that can be requested cannot exceed the NIH stipend level for 100% effort. If the trainee is contributing less than 100% effort then the stipend request must be reduced to correspond to this effort. For example, if the trainee’s effort is 85%, then the stipend requested can be no more than 85% of the appropriate NIH stipend level.
For Clinician Scientist Development Awards, the PI is required to contribute at least 50% effort to the proposed work.
For Graduate Research Fellowships, the only allowable budget items are the graduate student’s salary/stipend plus applicable fringe benefits (including health insurance) and tuition costs and fees that are not already covered by the institution.
If funded, the amount approved by FARA may be different from the requested budget. This change could be as a result of the LOI reviews and/or the full application reviews, or it may be a decision made by FARA when the award is approved.
Milestones
All projects must have objective milestones that are clearly communicated. We appreciate that some projects, especially those that are more basic-science-oriented, might need to have milestone adjustments based on specific experimental outcomes. If the proposal is funded, FARA will utilize the proposed milestones for scheduling progress reports and monitoring the project.
Human Subjects
If human subjects are used in the proposed study, the study must be approved by the Institutional Review Board (IRB) or equivalent. Full funding will not be provided until proof of IRB approval is demonstrated to FARA. Human subjects studied during research conducted under a FARA grant are under no circumstances a responsibility of FARA.
Animal Research
If animals are used, the proposed study must be approved by the Institutional Animal Care and Use Committee (IACUC), or equivalent, indicating that appropriate precautions have been taken to assure that proper treatment, care, and humane conditions are provided.
Other Documents
This can include all supporting materials such as appendices, institutional transmittal forms, letters of support, etc.