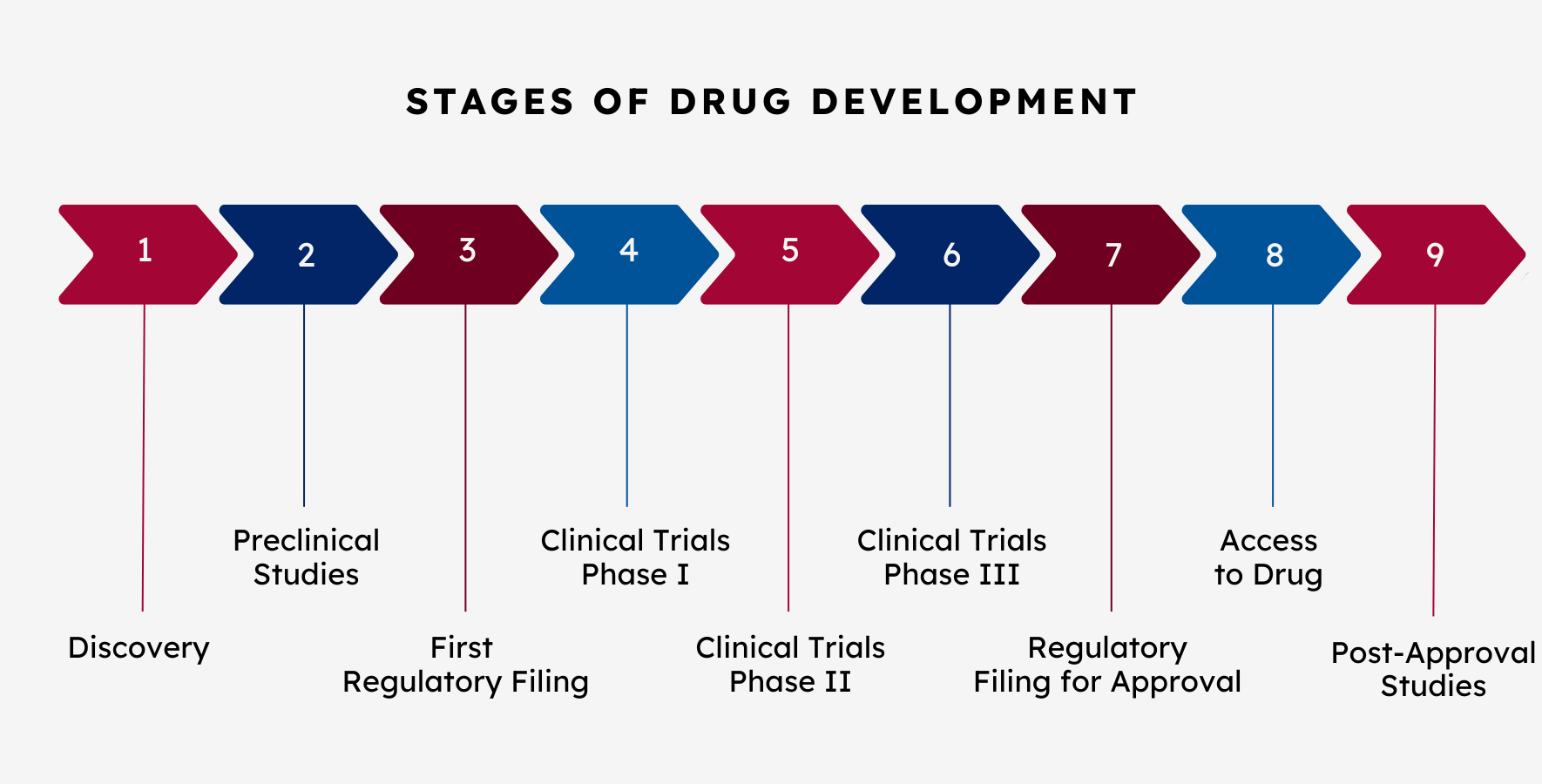
Stage 1 – Discovery
The discovery phase includes the identification or creation of a therapeutic that might be able to address the symptoms or underlying cause of a disease. For every 20,000-30,000 compounds tested in this first step, only one is eventually approved.
Stage 2 – Preclinical Studies
After a therapeutic is discovered, it must be tested in the laboratory on cell lines, animals, and other models of disease. Mandatory studies in model systems gather data on safety and efficacy of a drug that will help determine whether the drug may work in humans.
Stage 3 – First Regulatory Filing
When a sponsor feels like they have enough data to support human studies (clinical trials) for their therapeutic, they submit their preclinical data and plan for human studies to a regulatory agency. In the United States, this application to the Food and Drug Administration (FDA) is called an Investigation New Drug (IND), and in Europe, the application to the European Medical Association (EMA) is called a Clinical Trial Application (CTA). If the regulatory agency deems that the potential benefits of the therapeutic outweigh any known safety issues, they will allow the sponsor to proceed into the first of several clinical trials.
Stage 4 – Clinical Trials – Phase I
Clinical trials investigate the safety and efficacy of a therapeutic in humans. There are multiple phases of clinical trials. A Phase I clinical trial is the first time the therapeutic is in human studies, and it is done to determine safety, the correct dose, and sometimes early signs of efficacy. In the case of small molecule therapeutics, the Phase I trial is often in healthy volunteers. However, when the therapeutic is a protein or genetic medicine, the Phase I trial will usually require people with the condition that is being treated. Only 10-20% of therapeutics investigated in clinical trials ultimately get approved for use in patients.
Stage 5 – Clinical Trials – Phase II
A Phase II clinical trial is a larger study that is designed to determine the efficacy of the therapeutic in the intended treatment population.
Stage 6 – Clinical Trials – Phase III
A Phase III clinical trial is a large, multi-center study to confirm safety and efficacy in a large cohort of patients. It can provide the data for regulatory approval, and it is sometimes referred to as a pivotal trial.
Stage 7 – Regulatory Filing for Approval
If there is a clinical benefit and the therapeutic is found to be safe, approval can be requested from the regulatory agency to market and sell the therapeutic. This is called a New Drug Application (NDA) in the United States
However, if the therapeutic is a biologic (i.e. a protein, an antibody, gene therapy), then the application is called a Biologics Licensing Application (BLA) in the United States.
Both application types are called a Marketing Authorization Application (MAA) in the European Union.
Stage 8 – Access to Drug
If the therapeutic is approved by the Food and Drug Administration in the US and the European Commission in the European Union, the focus then moves to facilitating access to the treatment. This is done through manufacturing, distribution, educating providers, and working with health insurance/payors.
Stage 9 – Post-Approval Studies
Sometimes a regulatory agency (for example, the FDA or the EMA) may require a company to continuing studying a drug even after it has been approved and made available for patients. The goals of these post approval studies are to provide additional information on effectiveness or safety, including in specific patient groups, for example, those with other conditions.
Inactive
Therapeutic development programs become inactive when the proposed treatment failed to show benefit or had safety issues that cannot be overcome. Programs may also be paused when there is no clear path to obtain the resources needed to continue development.