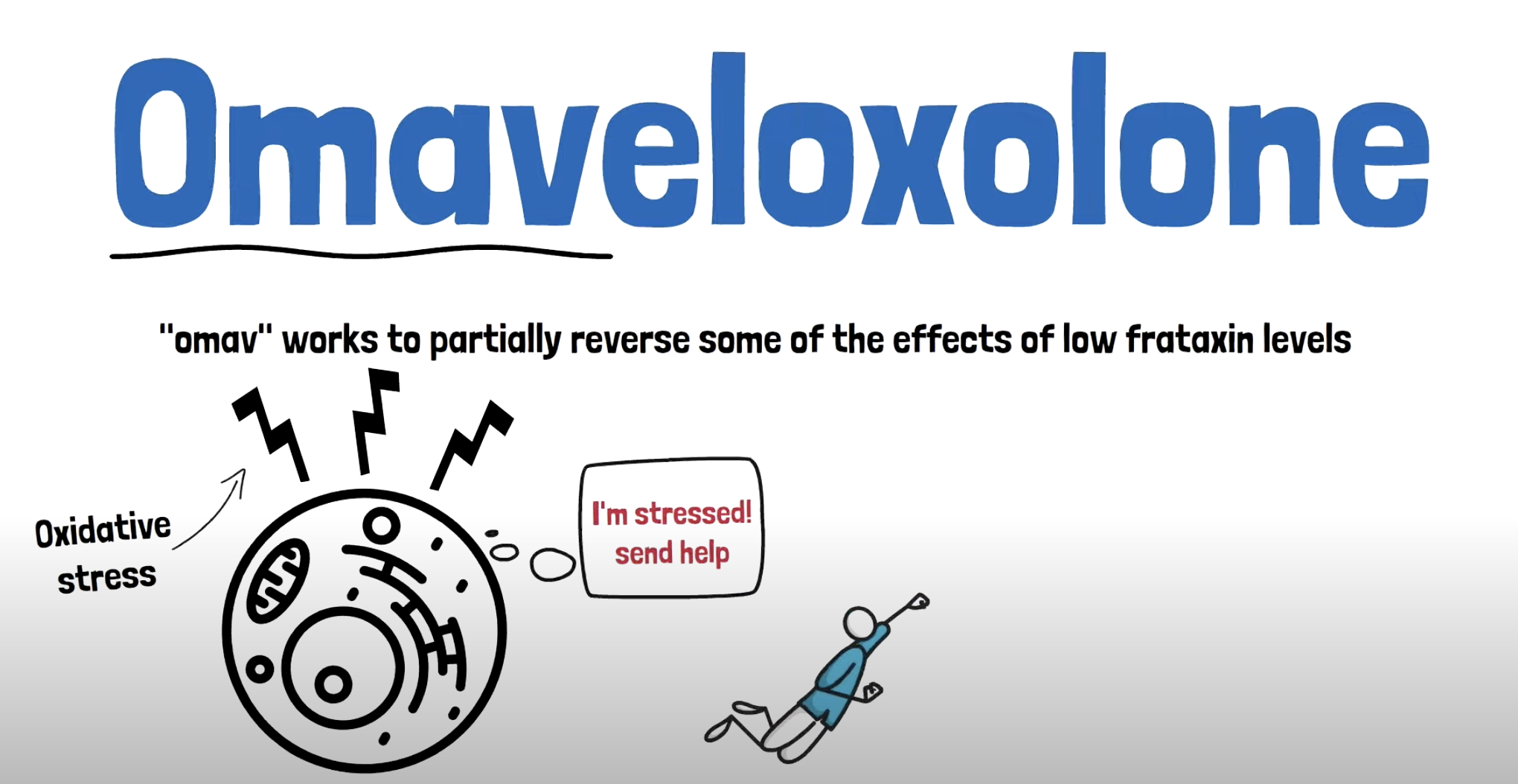
Two-Minute Mechanism Video: Omaveloxolone
This short video explains how omaveloxolone works to potentially provide benefit to someone with FA.
Omaveloxolone (aka omav), brand name SKYCLARYS™, is a small molecule that is delivered orally. It works by enhancing the activity of the transcription regulator, NRF2. On February 28, 2023, the United States Food & Drug Administration (FDA) approved SKYCLARYS™ in FA for people 16 years of age and older. This marked a milestone for the FA community- the first approved treatment for FA. The MOXIe clinical trial demonstrated a slowing of disease progression following treatment with omav.
* The drug is approved for use in the US, EU, Canada, UK, and Brazil for people with FA 16 years of age and older.
Additional clinical studies are underway to support the approval of use in people with FA younger than 16 years of age.
The drug development process can be thought of as a series of stages, and successful drugs must pass through each stage to become available to patients.
Several academic research labs demonstrated in both human FA cell models and mouse models that Nrf2 is downregulated. In addition, treatment of FA cell and animal models with omavoloxone demonstrated activation of Nrf2 and improvements in the mitochondrial function. Studies have shown that treatment with omav reduced pathologic levels of oxidative stress, restored antioxidative response, restored complex 1 activity, decreased lipid peroxidation, and decreased mitochondrial reactive oxygen species (ROS). In cellular assays, omav prevented cell death following pro-oxidant challenge. See links to published papers to learn more about these studies.
Working with FARA and several FA Investigators in our Collaborative Clinical Research Network (CCRN), Reata conducted a two-part, randomized, placebo-controlled Phase 2/3 Study of the Safety, Efficacy, and Pharmacodynamics of omav. Part 1 of the study was a randomized, placebo-controlled, double-blind, dose-escalation study. Part 2 of the study was conducted to establish the longer-term effectiveness of omav. Finally, Reata also conducted an open label extension study. People who participated in MOXIe part 1 or 2 were able to take omav and continue to be followed for assessment of both efficacy and safety.
Biogen is conducting two pediatric studies of omaveloxolone (BIIB141) in Friedreich’s ataxia: BOLD, an ongoing Phase I safety, tolerability, and pharmacokinetic (PK) study, and BRAVE, a global Phase 3 trial.
BOLD began in May 2024 to investigate dose-safety and pharmacokinetics (PK) of a single dose of BIIB141 in children with FA. It initially enrolled participants ages 12–15, later expanding to include ages 6–11 and, as of March 2025, ages 2–5. An additional higher-dose cohort is underway in 2025.
In June 2025, Biogen announced the launch the BRAVE study—a two-part, randomized, placebo-controlled trial with an open-label extension—to evaluate effects and long-term safety in children ages 2 to under 16. Both non-ambulatory and ambulatory participants may qualify for the study. BRAVE will include 6 U.S. sites and with a total of 27 sites globally. The recruitment status for each site is updated on the BRAVE clinical trial page.
February 28, 2023: Reata Pharmaceuticals announced that the United States Food & Drug Administration (FDA) has approved their New Drug Application (NDA) for SKYCLARYS™ (aka omaveloxolone or omav) in FA for people 16 years of age and older. This marks a milestone for the FA community- the first approved treatment for FA.
February 12, 2024: Biogen Inc. announced the European Commission (EC) has authorized SKYCLARYS® (omaveloxolone) for the treatment of Friedreich’s ataxia (FA) in adults and adolescents aged 16 years and older. SKYCLARYS is the first treatment approved within the European Union for this rare, genetic, progressive neurodegenerative disease.
March/April 2025: SKYCLARYS was approved by Health Canada on March 17, 2025 for the treatment of people with FA 16 years of age and older. Brazil’s regulatory agency, ANVISA, approved registration of SKYCLARYS for the treatment of the disease in patients over 16 years of age on April 11, 2025. On April 23, 2025 SKYCLARYS was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) as the first treatment for patients aged 16 years and older with Friedreich’s ataxia (FA) in the UK.
October 2, 2024:
In this study, researchers will learn more about the safety of BIIB141, also known as omaveloxolone or SKYCLARYS®. This is a drug available for doctors to prescribe for people with Friedreich’s Ataxia (FA). This is known as an “observational” study, which collects health information about study participants without changing their medical care. Participants for this study will be found using the Friedreich’s Ataxia Global Clinical Consortium (FA GCC). This is a group of study research centers that help provide clinical care for people with FA.
October 18, 2024:
SKYCLARYS® (Omaveloxolone) Pregnancy and Lactation Surveillance Program
A Post-Marketing, Observational, Descriptive Study to Assess the Risk of Pregnancy and Maternal Complications and Adverse Effects on the Developing Fetus, Neonate, and Infant Among Individuals Exposed to Omaveloxolone During Pregnancy and/or Lactation
The primary objective of the study is to assess the risks associated with pregnancy and maternal complications in women with Friedreich’s Ataxia (FA) exposed to omaveloxolone during pregnancy and/or lactation. The secondary objective is to assess the adverse effects on the developing fetus, neonate, and infant in women exposed to omaveloxolone during pregnancy and/or lactation.
This short video explains how omaveloxolone works to potentially provide benefit to someone with FA.