Improve Mitochondrial Function & Reduce Oxidative Stress
Omaveloxolone (Brand Name: SKYCLARYS for >16yo)
Biogen
Learn more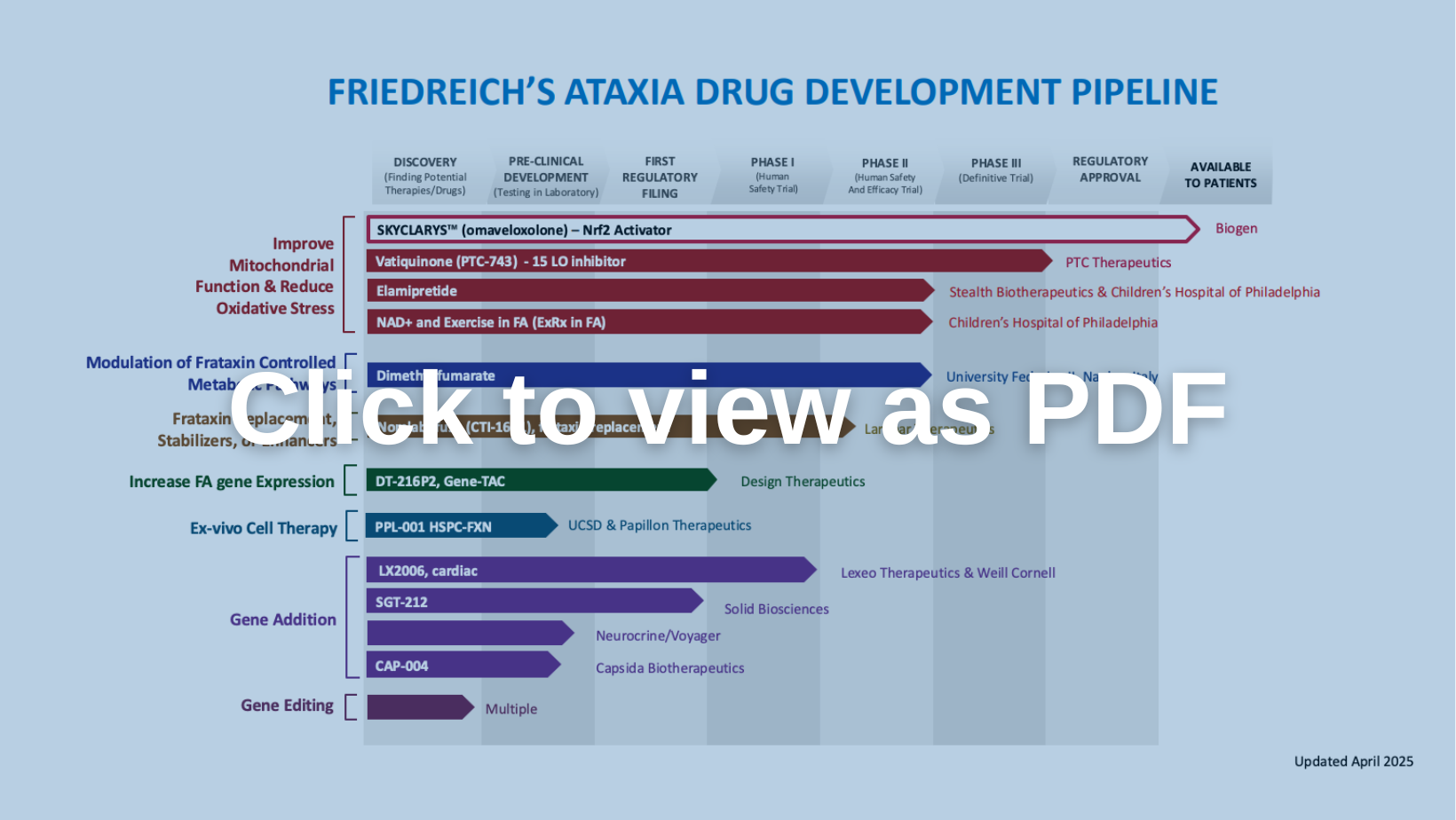
Improve Mitochondrial Function & Reduce Oxidative Stress
Biogen
Learn moreImprove Mitochondrial Function & Reduce Oxidative Stress
PTC Therapeutics
Learn moreImprove Mitochondrial Function & Reduce Oxidative Stress
Children’s Hospital of Philadelphia
Learn moreImprove Mitochondrial Function & Reduce Oxidative Stress
Children’s Hospital of Philadelphia
Learn moreModulation of Frataxin Controlled Metabolic Pathways
University of Federico II
Learn moreFrataxin Replacement, Stabilizers, or Enhancers
Larimar Therapeutics
Learn moreGene Addition
Neurocrine Biosciences / Voyager Therapeutics
Learn moreInactive
Berta Alemany, Institut d’Inverstigacio Biomedica de Girona
Learn moreInactive
Department of Neurology, Innsbruck Medical University, Innsbruck, Austria; Federico II University
Learn moreInactive
Richard Festenstein, Imperial College; Jorg Schulz, Aachen University
Learn more